Describe the Four Steps Used for Titration
Titration is an analytical chemistry technique used to find an unknown concentration of an analyte the titrand by reacting it with a known volume and concentration of a standard solution called the titrantTitrations are typically used for acid-base reactions and redox reactions. A solution of a strong base NaOH can be used as the standard solution in the titration but it needs to be standardized before titrating with an acid.
What Are The Pieces Of Apparatus Used In Titration Quora
The filtrate is evaporated to.
. Classification of Titrimetric Methods. Textbook 9th Edition Edit edition Solutions for Chapter 4 Problem 81RQ. Add 2-3 Drops of Brothymol Blue into flask 3.
Use a swirling motion to mix the solutions. Add 4-5 drops of phenolphthalein indicator. Take a burtte reading from the top of the miniscus.
So is gradually added to another solution. That is the type of titration described here. Excess solid metal metal oxide metal hydroxide or metal carbonate is added with stirring into a fixed volume of hot dilute acid.
Describe four steps needed to. Others Spectroscopy Titrations can also be used to furnish the purity of samples calculation regarding PH etc. Add a few drops of the appropriate indicator to the flask.
1 changing the pressure 2 changing the temperature 3 changing the concentration of mathrmH_2g 4 changing the concentration of HFg Verified answer CHEMISTRY. Use a swirling motion to mix the solutions. For any titration process the method is similar except for a few differences.
Describe the basic steps involved in an acid-base titration. 233 Triple only describe how to carry out an acid-alkali titration. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator.
Place a flask with a unknown concentration of acid or base under the burtte. Add 2-3 Drops of Universal Indicator into flask 2. No indicator is added to flask 4.
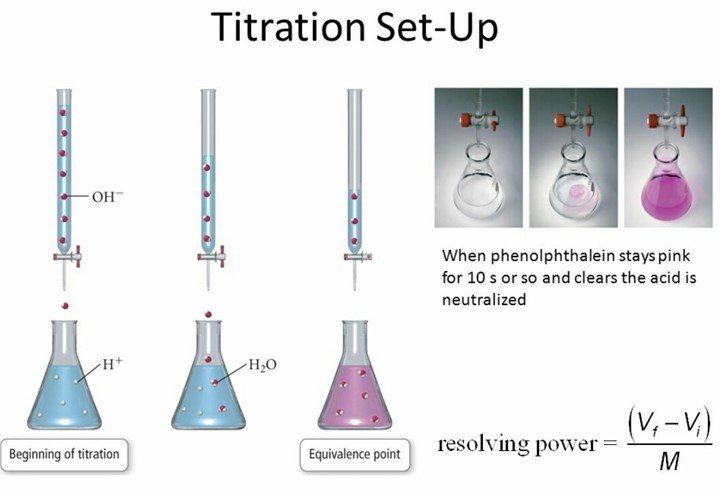
The diagram shows the titration method for a neutralisation reaction between hydrochloric acid and sodium hydroxide using phenolphthalein as an indicator. It is a common technique used for measuring amounts of acids and bases and is then called an acid-base titration. Titration is an important procedure because of its plays a key role in the quality of all the medications.
Repeat steps 1 to 5 until you. Place an aliquot of the dispensed acid solution 5-10 ml into a conical titration flask. Acidbase titration is used to determine the unknown concentration of an acid or base solution.
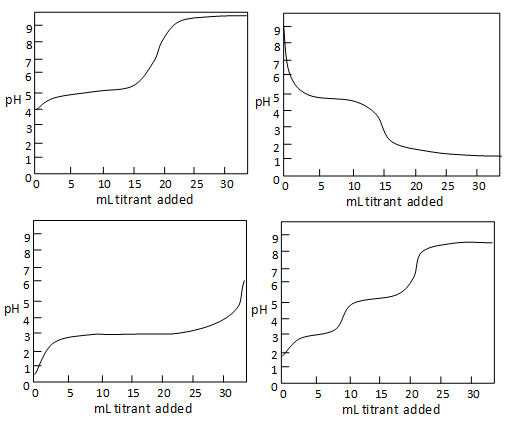
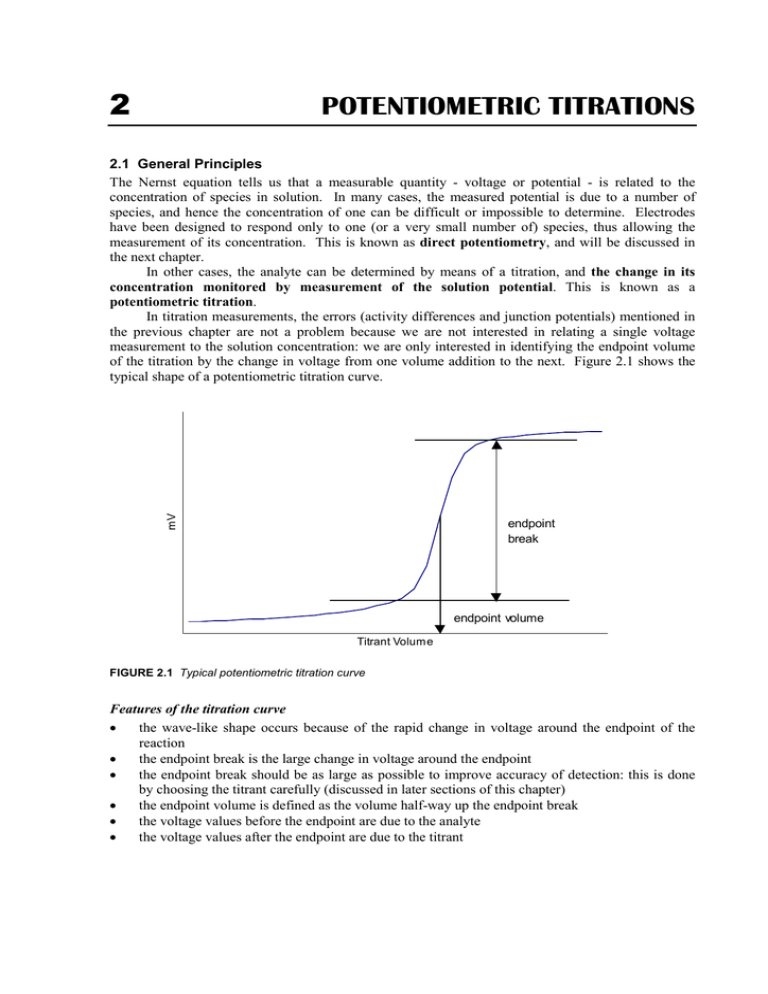
Fill a clean burette with a standard alkali solution and titrate the acid solution until a stable pink color appears. 2 The pH of the solution at equivalence point is dependent on the strength of the acid and strength of the base used in the titration. Why is this technique of great practical value.
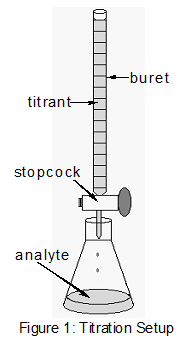
For determining unknown concentration of acid. A titration is a process in which a measured volume of a solution is added to a reaction mixture until some observable property has changed. Clamp the burette to your burette stand.
Prepare a standard solution of known concentration. The method used involves the following steps. For instance titration can be used to determine the levels of glucose in those diagnosed with diabetes.
Between the two solutions. Add 2-3 drops of the phenolphthalein indicator to the flask 1. Until the chemical reaction.
The titration process can be divided in the following way. Titration is used to find out precisely how much acid neutralises a certain volume of alkali or vice versa. Lab technicians often use titration when dealing with blood and urine samples from patients.
We are going to start with a solution of accurately known concentration. Fill the burtte with known concentration of an acid or base. 1 The equivalence point of an acid-base reaction the point at which the amounts of acid and of base are just sufficient to cause complete neutralization.
Fill your burette with titrant solution using the graduations on the instrument to precisely gauge the amount added. The unreacted solid is removed by filtration. Acid Base Titration 2.
Use a swirling motion to mix the solutions. Get an acid solution of unknown concentration. Lets first list the basic steps or an acid beast penetration.
The following steps describe the methods involved in a titration analysis using a visual indicator. Because titration is an analytical technique. This is referred to as our standard solution.
Use for the substances which are not water soluble or which are weak acids or bases. Titrimetric methods are classified into various types depending on the nature of chemical reactions.
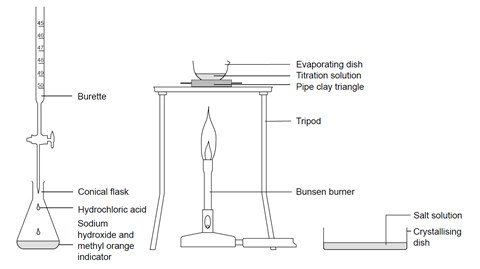
Titrating Sodium Hydroxide With Hydrochloric Acid Experiment Rsc Education

Titration Uses In Real Life Studiousguy
What Are The Essential Laboratory Apparatus For Titration Quora
17 3 Acid Base Titrations Chemistry Libretexts

Acid Base Titrations Introduction To Chemistry
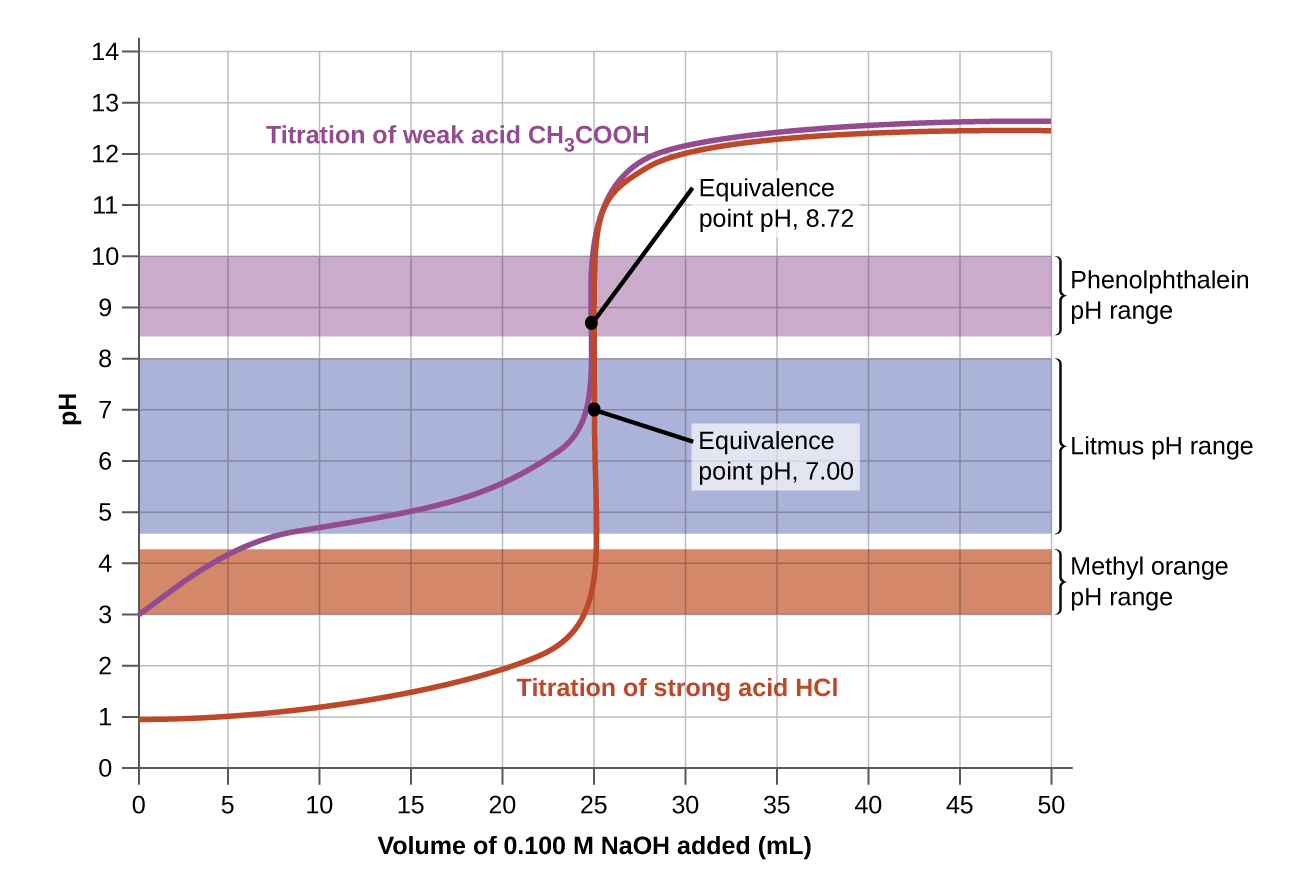
14 7 Acid Base Titrations Chemistry

How Is Titration Used In The Pharmaceutical Industry

Titration Curves Equivalence Point Article Khan Academy

Strong Acid Strong Base Titrations
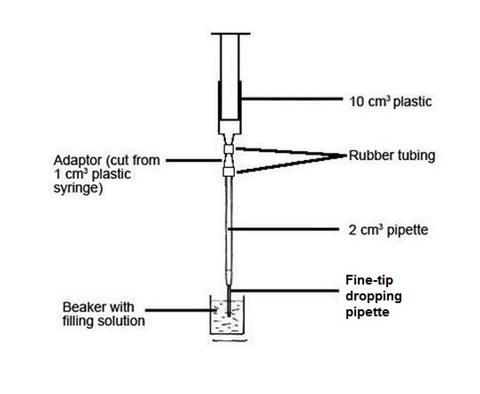
A Microscale Acid Base Titration Experiment Rsc Education
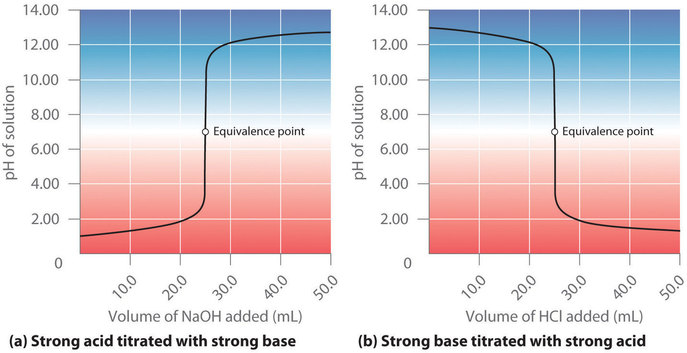
17 4 Titrations And Ph Curves Chemistry Libretexts

Titration Curves Equivalence Point Article Khan Academy
15 Uses Of Titration All Uses Of


Comments
Post a Comment